Bladder cancer is diagnosed in thousands of people in the UK each year. 70-80% of cases are non-muscle invasive (NMIBC), meaning it is localised in the bladder wall and has not spread. If it is caught early at this stage, the chances of successful treatment are significantly improved. However, even when bladder cancer is detected at an early stage and treated appropriately, it often recurs. It can recur anywhere along the urinary tract, kidneys, ureters, prostate or bladder. Therefore, close follow-up is required after treatment, which is done with regular cystoscopies.
Bladder EpiCheck®
How does Bladder EpiCheck® work?
Bladder EpiCheck® provides patients and clinicians with a simple urine test to detect early and recurring bladder tumours. The test analyses subtle disease-specific changes in DNA methylation markers, allowing for the detection of high-risk (non-Ta-LG) cancers. You receive a result with a clear positive or negative for presence of bladder cancer, with results available in 5-7 days.
Clinical Value
The test has a very high Negative Predictive Value (NPV) of 99.3%, meaning that a negative result correctly rules out bladder cancer 99.3% of the time and does not rely on human interpretation.
Clinical value of EpiCheck can be found in:
- Surveillance: Bladder EpiCheck® is recommended to be used in alternation with cystoscopies for surveillance, to reduce the number of cystoscopies required. A negative result would justify extension of cytoscopy intervals.
- Early detection: Bladder EpiCheck® is recommended for early detection of bladder cancer and upper tract urothelial carcinoma (UTUC), in patients presenting with hematuria and/or other urinary tract symptoms and/or findings with a suspicion of malignancy. Those with a positive test result would then be recommended for a cystoscopy, however those with a negative result could be safely deescalated, avoiding the need for invasive cystoscopy.
Bladder EpiCheck in the NHS
For the NHS to adopt any new test, it must be approved by NICE guidelines based on data from large-scale NHS clinical trials. Currently, Bladder EpiCheck® has been reviewed by NICE and an NHS trial is underway. As part of the review, expert advisors agreed that “the technology is not yet widely used in the NHS, but aside from cost, they were not aware of any major barriers to adoption”.
The first NHS trial commenced in Summer 2023 as a collaboration between Cambridge Clinical Laboratories, Nucleix, and Dr Mariappan at the Western General Hospital in Edinburgh. In this trial, patients who visit the clinic for regular cystoscopies for surveillance of bladder cancer are also doing a Bladder EpiCheck® test, and over the course of the trial EpiCheck® will replace up to half of all surveillance cystoscopies. The trial will enable cost-calculations to support the implementation of EpiCheck® into the NHS.
How EpiCheck Fits in the Patient Pathway
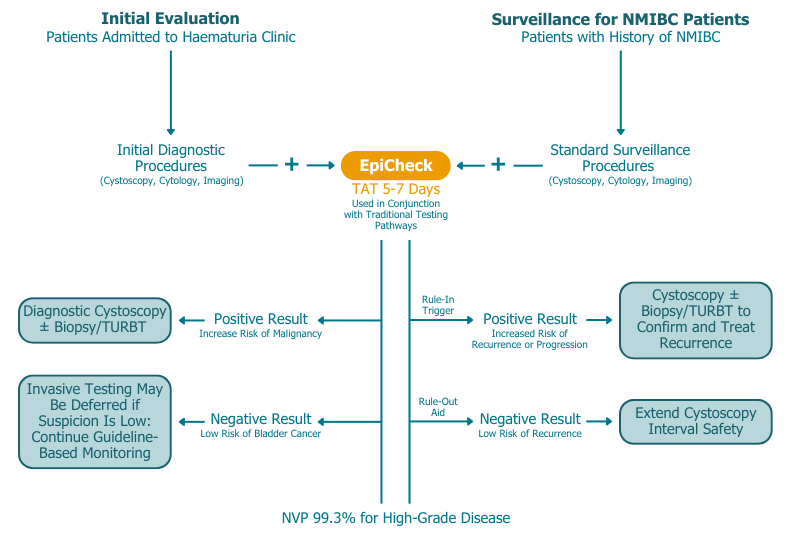
Bladder EpiCheck is best positioned as a surveillance tool for non-muscle invasive bladder cancer (NMIBC). It can be performed in parallel with standard follow-up procedures such as cystoscopy and cytology. With a negative predictive value (NPV) of 99.3%, a negative EpiCheck result confidently rules out high-grade recurrence and supports extending the interval between cystoscopies, reducing the frequency of this invasive procedure without compromising patient safety.
Partnering with Your Clinic to Enhance Patient Care
Source Genomics provides clinics and healthcare providers with access to novel biomarker-driven tests across multiple cancer types. Partnering with us will enable access to:
Source Genomics works with healthcare providers nationwide to integrate validated tests into patient pathways, improving outcomes and reducing invasive procedures. Speak with our clinical specialists today to learn more.