Prostate Cancer
Prostate cancer is one of the most frequently diagnosed cancers among men. Those whose disease is detected earlier show more favourable clinical outcomes. Genomic insights are transforming how this disease is diagnosed, monitored, and treated, offering a more personalised approach to patient care.
Prostate Testing
Proclarix
Proclarix is an easy, non-invasive blood test which analyses protein biomarkers combined with clinical variables (age, PSA, prostate volume) to assess the risk of aggressive prostate cancer, in those with a PSA between 2 and 10 ng/nL. A high-risk Proclarix score would indicate that you should go to the next stage of investigation, which is an MRI, whereas a low-risk score rules out prostate cancer with 95% accuracy. Proclarix can be used instead or alongside a standard PSA blood test with no additional intervention required, with results available within 5-7 working days.
PSA between 2 and 10 ng/nL is referred to as the PSA “grey area”, where it’s unclear if a raised PSA level indicates prostate cancer or not. Further, a PSA within these “normal” ranges doesn’t rule out prostate cancer, with 1 in 7 men with prostate cancer having a normal PSA. Proclarix provides clarity on these unclear PSA results by giving a more accurate and specific risk score for aggressive prostate cancer.
Stockholm3
Stockholm3 is an easy, noninvasive blood test for the early detection of prostate cancer. It measures the levels of 5 protein markers and the presence of multiple genetic markers. The result from Stockholm3 is a risk score that indicates the risk of aggressive prostate cancer as being either: elevated risk, normal risk or low risk. With elevated risk, a referral to a urologist for further examination is recommended, and with low or normal risk a new test within two to six years is recommended. Nearly half of the men aged 50 to 70 years have a low risk profile and do not need to take a new test until after six years. Stockholm 3 can be used instead or alongside a standard PSA blood test with no additional intervention required, with results available within 5-7 working days.
Early detection is the key for the successful treatment of prostate cancer. Stockholm3 finds high-risk men with normal PSA results, and furthermore finds twice as many men with aggressive prostate cancer requiring treatment compared to PSA in clinical routine. In addition using Stockholm3 before MRI minimises over-detection by identifying men at low risk of harmful prostate cancer, even if their PSA levels are elevated. For many men, this will mean avoiding unnecessary and uncomfortable procedures like a prostate biopsy. Stockhom3 has been shown to cut the number of unnecessary biopsies in half compared to PSA in clinical practice.
Stockholm3 has been used in clinical practice in Sweden, Norway and Finland since 2019, it is included in European and American prostate cancer guidelines and has been tested in trials on over 75,000 men. Several major healthcare providers in Europe are using Stockholm3 instead of PSA screening.
Prostatype
The Prostatype Genomic Classifier (PCG) is a novel genetic test performed on prostate biopsy tissue. It generates a genomic risk score that helps consultants distinguish between patients with aggressive prostate cancer, who may benefit from curative treatment, and those with non-aggressive disease, who may instead be safely monitored through active surveillance.
Current methods for assessing prostate cancer aggressiveness rely heavily on visual assessment and human interpretation. As a result, treatment decisions are often based on subjective information that may not fully reflect the true biology of the patient’s tumour. This can lead to misclassification- overtreating some patients while undertreating others.
Prostatype addresses this challenge by providing an objective, genomics-based risk score. This offers value to every stakeholder in the patient journey:
- Clinicians gain a more reliable tool to guide treatment decisions.
- Patients may be reclassified into active surveillance, reducing unnecessary exposure to invasive treatment and its potential side effects.
By integrating genomic insights with traditional clinical parameters, Prostatype helps clinicians and patients make more confident, personalised decisions between active surveillance and curative treatment.
How our Prostate Tests Fit in the Patient Pathway
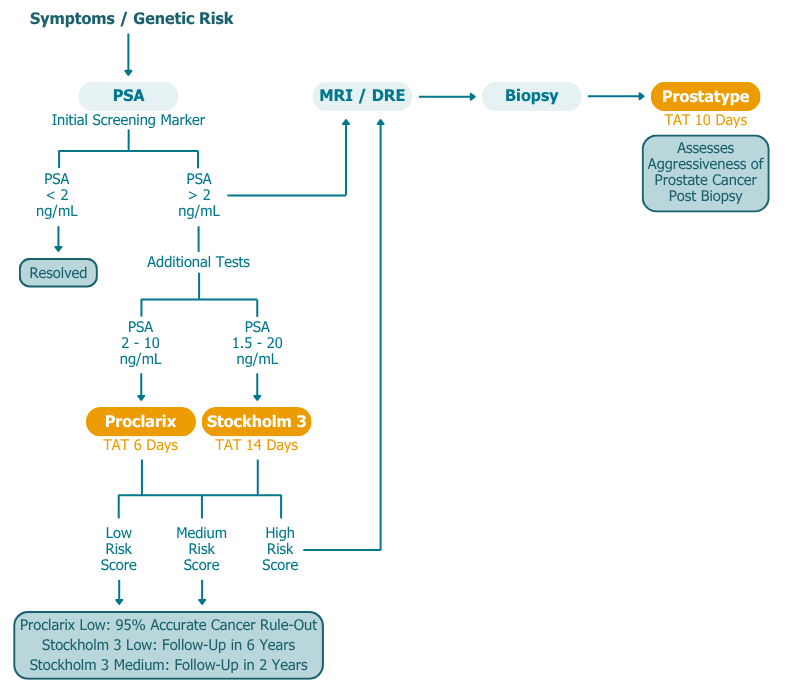
Proclarix and Stockholm3 serve as reflex tests for patients with PSA levels between 2–10 ng/mL, the PSA “grey zone.” These tests provide a more accurate assessment of an individual’s risk of clinically significant prostate cancer, helping to reduce unnecessary MRI scans and biopsies.
Prostatype, by contrast, is performed on post-biopsy tissue and provides a genomic risk score indicating the biological aggressiveness of prostate cancer. This helps clinicians distinguish candidates suitable for focal therapy from those who require more radical treatment.
Where to Access Prostate Cancer Tests
Proclarix
Goodbody Clinic Private Healthcare Testing
Home Test Kits
You can access Proclarix through a specialist prostate consultant by booking a consultation. During the appointment, the consultant will review your clinical data and family history, discuss any concerns, take a blood sample, and usually schedule a follow-up to go through the results. They can advise on any further monitoring or investigations if needed.
The consultants who currently provide Proclarix upon request are listed below. However, any private consultant or GP can also contact us to order a Proclarix test on your behalf.
Mr Ahmed Ali
London/Hampshire/Surrey
Urology Partners
Mr Jaimin Bhatt
Glasgow
Ross Hall Clinic
Mr Tim Dudderidge
Southampton
Spire Southampton
Professor Chris Eden
Santis Health in The Shard
Guildford Nuffield Hospital
Professor Mark Emberton
London
London Urology Specialists
Mr Stephen Gordon
Surrey
Spire St Anthony
Ms Maya Harris
Warwick
Nuffield Health
Mr Alan Doherty
Birmingham
Birmingham Prostate Clinic
Mr Sergey Tadtayev
Doctify
Asif Naseem GP
London
Dr Naseem
Private GP Healthcare
Ashford, Kent
Private gp health care
Innermost Healthcare
Cardiff, Wales
Innermost healthcare
Concierge Medical
Cotswolds and surrounding areas (Gloucestershire, Worcestershire, Wiltshire, Warwickshire and Oxfordshire)
Concierge Medical
The Private Doctors
Leeds
The Private Doctors
You can book in for a Proclarix test at a clinic local to you, where your blood sample will be taken. A short appointment may be required to ensure all your clinical data is collected. The clinic provider will then deliver your results via email, phone call or in-person appointment. The report containing your results describes what the risk score means. You can book in to discuss this result with a GP or consultant if required/preferred.
Remedi Health
Winchester
Remedi Health
Concierge Medical
Warwick
Concierge Medical
Mans Matters Clinic, Knightsbridge
Mans Matters
Shockwaves Clinic
Knightsbridge
Shockwaves Clinc
We are partnered with Innermost Healthcare who can organise home-delivery of the urine test kit and courier collection for the day that you do the sample. Please contact Innermost Healthcare to book this service.
Concierge Medical in Warwick also provide a home visit service in their area, Please contact Concierge Medical to book this service.
Stockholm3
Goodbody Clinic Private Healthcare Testing
Home Test Kits
You can access Stockholm3 through a specialist prostate consultant by booking a consultation. During this appointment, the consultant will review your clinical data, family history, and any concerns you may have. They will take your blood sample and usually arrange a follow-up consultation to discuss the results. The consultant will also advise you on any further monitoring or investigations if required.
The consultants who provide Stockholm3 upon request are listed below:
Mr Ahmed Ali
London/Hampshire/Surrey
Urology Partners
Mr Jaimin Bhatt
Glasgow
Ross Hall Clinic
Utsav Reddy
Norwich
Spire Healthcare
Mr Alan Doherty
Birmingham
Birmingham Prostate Clinic
Mr Sergey Tadtayev
Doctify
Asif Naseem GP
London
Dr Naseem
Private GP Healthcare
Ashford, Kent
Private gp health care
The Private Doctors
Leeds
The Private Doctors
You can book in for a Stockholm3 test at a clinic local to you, where your blood sample will be taken. A short appointment may be required to ensure all your clinical data is collected. The clinic provider will then deliver your results via email, phone call or in-person appointment. The report containing your results describes what the risk score means. You can book in to discuss this result with a GP or consultant if required/preferred.
Remedi Health
Winchester
Remedi Health
Mans Matters Clinic, Knightsbridge
Mans Matters
Shockwaves Clinic
Knightsbridge
Shockwaves Clinc
Partnering with Your Clinic to Enhance Patient Care
Source Genomics provides clinics and healthcare providers with access to novel biomarker-driven tests across multiple cancer types. Partnering with us will enable access to:
- Enhanced delivery of diagnostic testing for your patients, with a focus on personalised care.
- Fast turnaround times of test results.
- Accredited Quality Standards: All testing is performed in ISO-accredited laboratories with validated workflows and rigorous quality control.
Source Genomics partners with healthcare providers to integrate validated blood-based and genomic prostate cancer tests into patient pathways, from early risk assessment to treatment selection. Contact us today to connect with clinical specialists or request test implementation details.