Improve Patient Outcomes with ToxNav® – a More Comprehensive Personalised Approach to 5FU/Capecitabine Treatment
5-fluorouracil (5FU) and its prodrug, Capecitabine, are widely used to treat multiple types of cancer. However, they can cause severe side effects, and an estimated 1% of patients will die due to 5FU/Capecitabine therapy. ToxNav® is a genomic assay for predicting the risk of severe adverse reactions to 5FU/Capecitabine that offers several advantages over alternative tests. By using ToxNav® for Next Generation Sequencing of patient blood samples before administering 5FU/Capecitabine, potentially life-threatening toxicities could be prevented and the associated burden on the healthcare system reduced.
5FU/Capecitabine toxicity is linked to DPD deficiency
5FU and Capecitabine function by being catabolised to fluorouracil, a cytotoxic agent that prevents DNA synthesis and slows tumour growth. Dihydropyrimidine dehydrogenase (DPD) is the rate-limiting enzyme in this process, meaning that patients with a complete or partial DPD deficiency are at increased risk of experiencing a severe adverse reaction to 5FU/Capecitabine treatment. DPD deficiency is most often caused by inherited variants of the DPYD gene, which can be detected by screening for single nucleotide polymorphisms (SNPs). It is a clinical requirement for patients to undergo testing for DPD deficiency before receiving 5FU/Capecitabine, which is contraindicated in those with a complete DPD deficiency and should be administered at a reduced starting dose where a partial DPD deficiency is identified.
ToxNav® uses a panel of DPYD alleles to assess risk
Alternative laboratory developed tests and CE-marked kits for assessing DPD deficiency tend to detect just 4-5 SNPs. In contrast, ToxNav® uses 20 SNPs for superior specificity and sensitivity, including several markers that are known to be more prevalent and of higher impact in ethnic minority groups. This allows patients to be stratified into risk groups: critical risk, high risk, standard risk (with high risk of hand foot syndrome), and standard risk.
Crucially, ToxNav® is the only DPD deficiency test available that has undergone robust clinical validation in a large study. During this investigation, the selected panel of no/low function DPYD alleles was shown to have clinical significance in predicting severe 5FU/capecitabine-related adverse events.
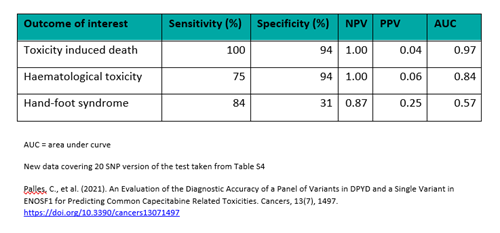
Clinical validation of ToxNav® in colorectal cancer. Using data from a Phase III randomised controlled clinical study (QUASAR 2), 20 genetic markers were selected for testing in patients with gastrointestinal toxicities and haematological toxicities. The chosen markers demonstrated high sensitivity and specificity, with the corresponding Negative Predictive Value (NPV) and Positive Predictive Value (PPV) data indicating clinical significance.
To find out more about ToxNav® and how it can be used to prevent severe adverse reactions to 5FU/Capecitabine, contact Enquiries@sourcebioscience.com or +44 (0) 115 973 9012


Contact us today and one of our skilled account managers will be in touch with a free consultation including further information and pricing details.
Share this article

