Promoting Molecular Pathway Testing for Targeted Treatment of Colorectal Cancer
Colorectal Cancer as a Leading Cancer Type in the UK
With over 42,000 diagnoses made each year, colorectal cancer (CRC) is recognised as a significant contributor to the ever-growing cancer caseload across the UK1. While most cases of CRC occur sporadically, around 5-10% of CRC cases are derived from inherited cancer syndromes2, such as Lynch Syndrome, where an estimated 1 in every 300 people in the UK may be carriers of a germline gene mutation associated with LS3.
Targeted treatment options for metastatic CRC (mCRC) have been recommended and licenced in the UK for more than a decade, primarily through DNA testing of specific mutations within the KRAS gene known to predict response to anti-EGFR monoclonal antibody therapies. This testing expanded over time to include additional targets within KRAS and within the NRAS gene. Mutation testing of specific regions of the BRAF gene is also clinically beneficial as a prognostic tool and predictive biomarker.
Since 2017, the National Institute of Health and Care Excellence (NICE) has recommended that diagnostic testing capabilities are expanded to allow all patients with CRC to be tested for Lynch syndrome5. In widening genetic testing procedures for CRC cases, a higher degree of LS cases can be identified, and better patient outcomes can be established through early disease detection5.
Lynch Syndrome Significantly Increases the Risk of Colorectal Cancer
Lynch syndrome (LS), also known as Hereditary Non-Polyposis Colorectal Cancer (HNPCC), is a hereditary syndrome whereby a genetic predisposition to various cancer types is observed1. Genetically, LS is most commonly caused by a mutation in one of four DNA mismatch repair (MMR) genes: MLH1, MSH2, MSH6 or PMS25. MMR proteins function to recognise and repair base-pairing errors throughout cell replication, and so mutations in any one of these genes can significantly impair the functioning of the MMR system – fostering defective base pairing during cell replication5. This makes LS a high genetic influence on cancer.
The Genetic Breakdown of Lynch Syndrome-associated CRC
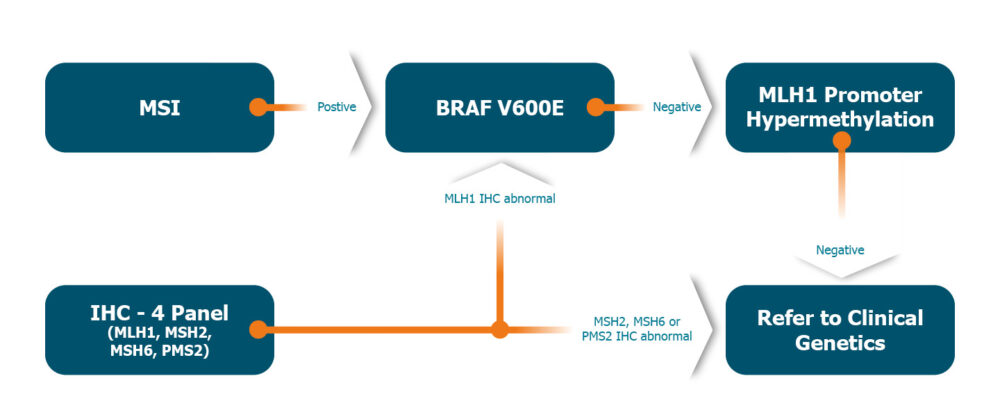
Patients with CRC who do not have an effective MMR system present a greater degree of replication errors in microsatellites – repetitive sequences of DNA with a high risk of genetic diversity – causing these fragments to fluctuate in length. This is known as microsatellite instability (MSI)5. MSI testing is therefore an effective first-line molecular testing strategy for identifying abnormalities in the MMR system of a patient’s tumour5.
It’s important to acknowledge — however —that deficient MMR systems are also seen in patients with sporadic CRC, meaning the use of wider biomarker testing for optimising diagnostic accuracy and downstream treatment plans becomes a critical consideration for CRC patients. In MSI-positive CRC cases, molecular testing for the BRAF V600E variant and MLH1 promoter methylation are effective in differentiating between sporadic CRC and LS tumours, and to subsequently identify patient specificity to treatment. Therefore, both BRAF mutation testing and MLH1 hypermethylation analysis broaden the scope for a Lynch syndrome-associated pathway testing to aid effective targeted treatment of individuals.
Using Pathway Testing to Identify Genetic Predisposition to Cancer Progression from Lynch Syndrome
As the field of precision medicine is constantly expanding, there is an increasing number of clinically actionable biomarkers available for clinicians to guide patient treatment pathways for improved clinical outcomes. Most significantly, the field of molecular diagnostic testing for cancer is undergoing a shift in the way it is deployed – moving away from single-test requests and towards pathway testing.
An ambition set by the NHS Long Term Plan states that 75% of cancers will be subjected to early diagnosis, by 20286. Although LS is not a direct initiator of cancer, around half of those diagnosed with LS later develop CRC, meaning the implementation of LS-related testing pathways on a national scale will be significant in reaching the ambitions set out by the NHS England in their aim to diagnose cancer early in anticipation of driving personalised treatment of patients.
Pharmacogenomic testing to predict drug toxicity in Colorectal Cancer
5-flurouracil (5FU) and its prodrug, capecitabine, are widely used in the clinical setting to treat multiple cancer types, however an estimated 1% of patients will have a fatal response to 5FU/capecitabine therapy as a result of its potential to cause severe adverse side effects.
5FU and capecitabine function by being catabolised to fluorouracil, a cytotoxic agent that prevents DNA synthesis and slows tumour growth. Dihydropyridine dehydrogenase (DPD) is the rate-limiting enzyme in this process, meaning that patients with a complete or partial DPD deficiency are at increased risk of experiencing a severe adverse reaction to 5FU/Capecitabine treatment.
CRC with a high presence of MSI has more recently been associated with a lack of benefit from adjuvant treatment with 5FU chemotherapy4. This makes the MSI biomarker a key forecaster of sensitivity to immunotherapy-based treatments for CRC following the diagnostic testing process.
Critically, ToxNav® is the only DPD deficiency test available that has undergone robust clinical validation in a large-scale study7. Read our previous blog post here to learn more about the importance of toxicity testing when directing cancer treatment plans and the utility of ToxNav® as a leading patient specificity test for precision therapeutics.
- Source BioScience provides an expansive portfolio of diagnostic tests to diagnose and monitor disease, detect risk, and identify appropriate therapy options for individual patient needs. For more information on how we can support, visit our website or get in touch today at enquiries@sourcebioscience.com
.References
- https://www.royalmarsden.nhs.uk/your-care/cancer-types/gastrointestinal/lower-gastrointestinal/colorectal-cancer
- https://www.ncbi.nlm.nih.gov/books/NBK431096/#:~:text=Most%20colorectal%20cancers%20occur%20sporadically,colorectal%20cancer%20syndrome%20(HNPCC).
- https://www.cancer.net/cancer-types/lynch-syndrome
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3612054/
- https://www.nice.org.uk/guidance/dg27/resources/molecular-testing-strategies-for-lynch-syndrome-in-people-with-colorectal-cancer-pdf-1053695294917
- https://www.england.nhs.uk/cancer/strategy/#:~:text=The%20key%20ambitions%20in%20the,(stage%20one%20or%20two).
- https://pubmed.ncbi.nlm.nih.gov/33805100/
Contact us today and one of our skilled account managers will be in touch with a free consultation including further information and pricing details.
Share this article

